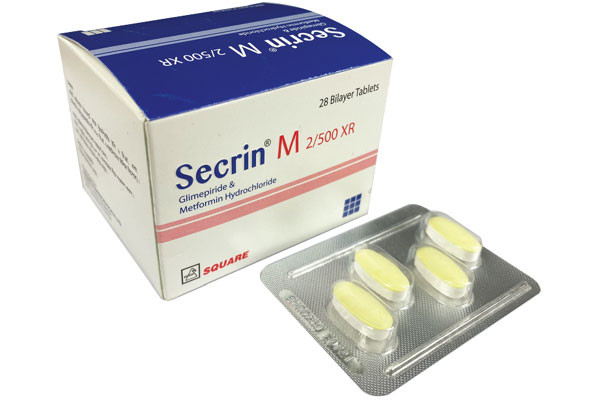

Secrin M Tablet (Extended Release), Glimepiride + Metformin 2 mg+500 mg
Inhouse product
-
৳11.40
৳12.00 -
৳42.75
৳45.00 -
৳16.63
৳17.50 -
৳2.14
৳2.25
Reviews & Ratings
Indications
Secrin M tablet is
indicated as an adjunct to diet and exercise in type 2 diabetes mellitus
patients-
- In case that the monotherapy
with glimepiride or metformin does not result in adequate glycemic
control.
- Replacement of combination
therapy of glimepiride and metformin.
* রেজিস্টার্ড চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন'
Pharmacology
Glimepiride is a
sulfonylurea antidiabetic agent which decreases blood glucose concentration.
The primary mechanism of action of Glimepiride appears to be dependent on
stimulating the release of insulin from functioning pancreatic beta cells.
Glimepiride acts in concert with glucose by improving the sensitivity of beta
cells to physiological glucose stimulus, resulting in insulin secretion. In
addition, extrapancreatic effects like reduction of basal hepatic glucose
production, increased peripheral tissue sensitivity to insulin and glucose
uptake may also play role in the activity of Glimepiride. In non-fasting
diabetic patients, the hypoglycaemic action of a single dose of Glimepiride
persists for 24 hours.
Metformin Hydrochloride is a biguanide type oral antihyperglycemic drug used in
the management of type 2 diabetes. It lowers both basal and postprandial plasma
glucose. Its mechanism of action is different from those of sulfonylureas and
it does not produce hypoglycemia. Metformin Hydrochloride decreases hepatic
glucose production, decreases intestinal absorption of glucose and improves
insulin sensitivity by an increase in peripheral glucose uptake and
utilization.
Dosage
- The dosage of this tablet is
governed by the desired blood glucose level. The dosage of this tablet
must be the lowest which is sufficient to achieve the desired metabolic
control. During treatment with this tablet glucose levels in blood and
urine must be measured regularly.
- Mistakes, e.g. forgetting to
take a dose, must never be corrected by subsequently taking a larger dose.
- As an improvement in control of
diabetes is, in itself, associated with higher insulin sensitivity,
glimepiride requirements may fall as treatment proceeds. To avoid
hypoglycaemia timely dose reduction or cessation of this tablet therapy
must therefore be considered.
- The highest recommended dose
per day should be 8 mg of glimepiride and 2000 mg of metformin.
- In order to avoid hypoglycaemia
the starting dose of this tablet should not exceed the daily doses of
glimepiride or metformin already being taken.
- When switching from combination
therapy of glimepiride plus metformin as separate tablets, this
combination should be administered on the basis of dosage currently being
taken.
* রেজিস্টার্ড চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন'
Administration
This tablet must be
swallowed whole and not crushed or chewed.
* রেজিস্টার্ড চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন'
Interaction
For Glimepiride:
- Glimepiride is metabolized by
cytochrome P450 2C9 (CYP2C9). This should be taken into account when
glimepiride is coadministered with inducers (e.g. rifampicin) or
inhibitors (e.g. fuconazole) of CYP 2C9.
- Potentiation of the
blood-glucose-lowering efect and, thus, in some instances hypoglycaemia
may occur when one of the following drugs is taken, for example: insulin
and other, oral antidiabetics; ACE inhibitors; anabolic steroids and male
sex hormones; chloramphenicol; coumarin derivatives; cyclophosphamide;
disopyramide; fenfuramine; fenyramidol; fbrates; fuoxetine; guanethidine;
ifosfamide; MAO inhibitors; miconazole; fuconazole; para-aminosalicylic
acid; pentoxifylline (high dose parenteral); phenylbutazone; azapropazone;
oxyphenbutazone; probenecid; quinolones; salicylates; sulfnpyrazone;
clarithromycin; sulfonamide antibiotics; tetracyclines; tritoqualine;
trofosfamide.
- Weakening of the
blood-glucose-lowering efect and, thus raised blood glucose levels may
occur when one of the following drugs is taken, for example:
acetazolamide; barbiturates; corticosteroids; diazoxide; diuretics;
epinephrine (adrenaline) and other sympathomimetic agents; glucagon;
laxatives (after protracted use); nicotinic acid (in high doses);
oestrogens and progestogens; phenothiazines; phenytoin; rifampicin;
thyroid hormones.
- H2 receptor antagonists,
beta-blockers, clonidine and reserpine may lead to either potentiation or
weakening of the blood-glucose-lowering efect. Under the infuence of
sympatholytic drugs such as beta-blockers, clonidine, guanethidine and
reserpine, the signs of adrenergic counter-regulation to hypoglycaemia may
be reduced or absent.
- Both acute and chronic alcohol
intake may potentiate or weaken the blood-glucose-lowering action of
glimepiride in an unpredictable fashion.
- The efect of coumarin
derivatives may be potentiated or weakened.
- Bile acid sequestrant:
Colesevelam binds to glimepiride and reduces glimepiride absorption from
the gastrointestinal tract. Glimepiride should be administered at least 4
hours prior to colesevelam.
For Metformin:
Concomitant use not recommended:
- Alcohol: Alcohol intoxication is associated with an increased
risk of lactic acidosis, particularly in case of fasting, malnutrition or
hepatic insufciency. Avoid consumption of alcohol and alcohol-containing
medications
- Iodinated
contrast agents: Metformin must be
discontinued prior to, or at the time of the imaging procedure and not
restarted until at least 48 hours after, provided that renal function has
been re-evaluated and found to be stable.
- Combinations
requiring precautions for use:
Some medicinal products can adversely afect renal function which may
increase the risk of lactic Acidosis. When starting or using such products
in combination with metformin, close monitoring of renal function is
necessary. Glucocorticoids, beta-2-agonists, and diuretics have intrinsic
hyperglycemic activity. Inform the patient and perform more frequent blood
glucose monitoring. ACE-inhibitors may decrease the blood glucose levels.
Metformin may decrease the anticoagulant efect of phenprocoumon.
Therefore, a close monitoring of the INR is recommended. Levothyroxine can
reduce the hypoglycemic efect of metformin. Monitoring of blood glucose
levels is recommended, especially when thyroid hormone therapy is
initiated or stopped, and the dosage of metformin must be adjusted if
necessary.
Organic cation
transporters (OCT): Metformin is a substrate of both transporters OCT1 and
OCT2. Co-administration of metformin with:
- Inhibitors of OCT1 (such as
verapamil) may reduce efcacy of metformin.
- Inducers of OCT1 (such as
rifampicin) may increase gastrointestinal absorption and efcacy of
metformin.
- Inhibitors of OCT2 (such as
cimetidine, dolutegravir, ranolazine, trimethoprime, vandetanib,
isavuconazole) may decrease the renal elimination of metformin and thus
lead to an increase in metformin plasma concentration.
- Inhibitors of both OCT1 and
OCT2 (such as crizotinib, olaparib) may alter efcacy and renal elimination
of metformin.
Caution is therefore
advised, especially in patients with renal impairment, when these drugs are
coadministered with metformin, as metformin plasma concentration may increase.
If needed, dose adjustment of metformin may be considered as OCT
inhibitors/inducers may alter the efcacy of metformin.
Contraindications
For
Glimepiride-
- In patients hypersensitive to
glimepiride, metformin, other sulfonylureas, other sulfonamides, or any of
the excipients of Amaryl M.
- In pregnant women.
- In breastfeeding women.
No experience has been
gained concerning the use of glimepiride in patients with severe impairment of
liver function and in dialysis patients. In patients with severe impairment of
hepatic function, change-over to insulin is indicated, not least to achieve
optimal metabolic control.
For Metformin-
- Hypersensitivity to metformin
or any of the excipients.
- Any type of acute metabolic
acidosis such as lactic acidosis Diabetic ketoacidosis, diabetic pre-coma.
- Severe Renal failure or renal
disfunction (e.g., serum creatine levels >135 μmol/L in males and
>110 μmol/L in females), GFR < 30 mL/min.
- Acute conditions with the
potential to alter renal function such as Dehydration, severe infection,
intravascular administration of iodinated contrast agents etc.
- Acute or chronic disease which
may cause tissue hypoxia such as cardiac or respiratory failure, recent
myocardial infarction, shock
- Hepatic insufciency.
- Acute alcohol intoxication,
alcoholism.
- Lactation.
Side Effects
For
Glimepiride:
Metabolism and nutrition disorders-
- As a result of the
blood-glucose-lowering action of glimepiride, Hypoglycaemia which may also
be prolonged.
- The clinical picture of a
severe hypoglycaemic attack may resemble that of a stroke.
Eye disorders:
Especially at the start of treatment, there may be temporary visual impairment
due to the change in blood glucose levels. The cause is a temporary alteration
in the turgidity and hence the refractive index of the lens, this being
dependent on blood glucose level.
Gastrointestinal disorders-
- Occasionally, Gastrointestinal
symptoms such as nausea, vomiting, sensations of pressure or fulness in
the epigastrium, abdominal pain and diarrhea may occur.
- In isolated cases, there may be
hepatitis, elevation of liver enzyme levels and/or cholestasis and jaundice,
which may progress to life-threatening liver failure.
- Dysgeusia (frequency not known)
Blood and lymphatic
system disorders-
- Changes in the blood picture
may occur: Rarely, thrombocytopenia and, in isolated cases, leucopenia,
hemolytic anemia, erythrocytopenia, granulocytopenia, agranulocytosis or
pancytopenia may develop. Cases of severe thrombocytopenia with platelet
count less than 10,000/μl and thrombocytopenic purpura have been reported
in post-marketing experience (frequency not known).
Skin and subcutaneous
tissue disorders: Alopecia (frequency not known)
General disorders-
- Occasionally, Allergic or
pseudo allergic reactions may occur, e.g. in the form of itching,
urticaria or rashes. Such mild reactions may develop into serious
reactions with dyspnoea and a fall in blood pressure, sometimes
progressing to shock.
- In isolated cases, a decrease
in serum sodium concentration and allergic vasculitis or hypersensitivity
of the skin to light may occur.
Investigations:
Glimepiride, like all sulfonylureas, can cause weight gain (frequency not
known)
For Metformin:
- Gastrointestinal symptoms such
as nausea, vomiting, diarrhoea, abdominal pain and loss of appetite
(>10%) are very common. These occur most frequently during initiation
of therapy and resolve spontaneously in most cases.
- Metallic taste (3%) is common
- Decrease of vitamin B12
absorption with decrease of serum levels has been observed in patients
treated long-term with metformin and appears generally to be without
clinical signifcance (<0.01%). However, Cases of peripheral neuropathy
in patients with vitamin B12 defciency have been reported in
post-marketing experience (frequency not known). (frequency unknown)
- Lactic acidosis (0.03
cases/1000 patient-years) is very rare
- Hemolytic anemia (frequency
unknown)
- Reduction of thyrotropin level
in patients with hypothyroidism (frequency unknown)
- Hypomagnesemia in the context
of diarrhea (frequency unknown)
- Encephalopathy (frequency
unknown)
- Photosensitivity (frequency
unknown)
- Hepatobiliary disorders:
Reports of liver function tests abnormalities and hepatitis resolving upon
metformin discontinuation
Pregnancy &
Lactation
Pregnancy-
- For Glimepiride: Glimepiride must not be taken during pregnancy.
Otherwise, there is risk of harm to the child. The patient must change
over to insulin during pregnancy. Patients planning a pregnancy must
inform their physician. It is recommended that such patients change over
to insulin.
- For Metformin: When the patient plans to become pregnant and during
pregnancy, diabetes should not be treated with metformin but insulin
should be used to maintain blood glucose levels as close to normal as
possible in order to lower the risk of fetal malformations associated with
abnormal blood glucose levels.
Lactation-
- For Glimepiride: To prevent possible ingestion with the breast milk
and possible harm to the child, glimepiride must not be taken by
breast-feeding women. If necessary the patient must change over to
insulin, or must stop breastfeeding.
- For Metformin: Metformin is excreted into milk in lactating rats.
Similar data is not available in humans and a decision should be made
whether to discontinue nursing or to discontinue metformin, taking into
account the importance of the compound to the mother.
Precautions &
Warnings
For Glimepiride: In the initial weeks of treatment, the risk
of hypoglycemia may be increased and necessitates especially careful
monitoring. If risk factors for hypoglycemia are present, it may be necessary
to adjust the dosage of glimepiride or the entire therapy. This also applies
whenever illness occurs during therapy or the patient's life-style changes. It
is known from other sulfonylureas that, despite initially successful
countermeasures, hypoglycaemia may recur. Patients must, therefore, remain
under close observation. Severe hypoglycaemia further requires immediate
treatment and follow-up by a physician and, in some circumstances, in-patient
hospital care. Treatment of patients with G6PD-defciency with sulfonylurea
agents can lead to hemolytic anaemia. Since glimepiride belongs to the class of
sulfonylurea agents, caution should be used in patients with G6PD-defciency and
a non-sulfonylurea alternative should be considered.
For Metformin: Regular monitoring of thyroid-stimulating
hormone (TSH) levels is recommended in patients withhypothyroidism. Long-term
treatment with metformin has been associated with a decrease in vitamin B12
serumlevels which may cause peripheral neuropathy. Monitoring of the vitamin
B12 level is recommended.
Use in Special
Populations
Children: Data is insufficient to recommend pediatric
use of this tablet.
Renal impairment: A GFR should be assessed before initiation
of treatment with metformin-containing products and at least annually
thereafter. In patients at increased risk of further progression of renal
impairment and in the elderly, renal function should be assessed more
frequently, e.g. every 3-6 months. The maximum daily dose of metformin should
preferably be divided into 2-3 daily doses. Factors that may increase the risk
of lactic acidosis should be reviewed before considering the initiation of
metformin in patients with GFR<60 mL/min. If no adequate strength of this
tablet is available, individual monocomponents should be used instead of the
fixed dose combination.
GFR 60-89 ml/min:
- Metformin: Maximum daily dose is 3000 mg. Dose reduction
may be considered in relation to declining renal function.
- Glimepiride: The highest recommended dose per day should be 8 mg
of glimepiride.
GFR 45-59 ml/min:
- Metformin: Maximum daily dose is 2000 mg. The starting dose is
at most half of the maximum dose.
GFR 30-44 ml/min:
- Metformin: Maximum daily dose is 1000 mg. The starting dose is
at most half of the maximum dose.
GFR <30 ml/min:
- Metformin: Metformin is contraindicated
- Glimepiride: Change-over to insulin is indicated, not least to
achieve optimal metabolic control.
Overdose Effects
For Glimepiride: Acute overdosage, as well as long-term
treatment with too high a dose of glimepiride, may lead to severe
life-threatening hypoglycaemia. As soon as an overdose of glimepiride has been
discovered, a physician must be notifed without delay. The patient must
immediately take sugar, if possible in the form of glucose unless a physician
has already undertaken responsibility for treating the overdose. Careful
monitoring is essential until the physician is confdent that the patient is out
of danger. It must be remembered that hypoglycaemia may recur after initial
recovery. Admission to hospital may sometimes be necessary even as a
precautionary measure. In particular, signifcant overdoses and severe reactions
with signs such as loss of consciousness or other serious neurological
disorders are medical emergencies and require immediate treatment and admission
to hospital. If, for example, the patient is unconscious, an intravenous
injection of concentrated glucose solution is indicated (for adults starting
with 40 ml of 20% solution, for example). Alternatively in adults,
administration of glucagon, e.g. in doses of 0.5 to 1 mg i.v., s.c. or i.m.,
may be considered. In particular when treating hypoglycaemia due to accidental
intake of glimepiride in infants and young children, the dose of glucose given
must be very carefully adjusted in view of the possibility of producing
dangerous hyperglycaemia, and must be controlled by close monitoring of blood
glucose. Patients who have ingested life-threatening amounts of glimepiride
require detoxifcation (e.g. by gastric lavage and medicinal charcoal). After
acute glucose replacement has been completed it is usually necessary to give an
intravenous glucose infusion in lower concentration so as to ensure that the
hypoglycaemia does not recur. The patient's blood glucose level should be
carefully monitored for at least 24 hours. In severe cases with a protracted
course, hypoglycaemia, or the danger of slipping back into hypoglycaemia, may
persist for several days.
For Metformin: Hypoglycaemia has not been seen with
metformin doses of up to 85 g, although Lactic acidosis has occurred in such
circumstances. High overdose or concomitant risks of metformin may lead to
lactic acidosis Lactic acidosis is a medical emergency and must be treated in
hospital. The most efective method to remove lactate and metformin is
haemodialysis. Pancreatitis may occur in the context of a metformin overdose.
Therapeutic Class
Combination Oral
hypoglycemic preparations
Storage Conditions
Store in a cool (not
exceeding 25°C) and dry place, protected from light.
Frequently Bought Products
Regulose Oral Solution 100 ml bottle, Lactulose 3.35 gm/5 ml
Flamfix Tablet, Nabumetone 500 mg
Sonap Tablet, Naproxen Sodium 250 mg
Deflacort Tablet, Deflazacort 6 mg
A-Flox Capsule, Flucloxacillin Sodium 500 mg
Anzitor Tablet , Atorvastatin Calcium 40 mg
Product Queries (0)
Login Or Registerto submit your questions to seller
Other Questions
No none asked to seller yet
-
৳11.40
৳12.00 -
৳42.75
৳45.00 -
৳16.63
৳17.50 -
৳2.14
৳2.25






![Hepavir Tablet, Lamivudine [For Chronic Hepatitis B] 100 mg](https://www.skpharma.com.bd/public/uploads/all/kspg2JCX5MIlxuFb5mW5K4N9hAQH8ozC55rIAcdW.webp)