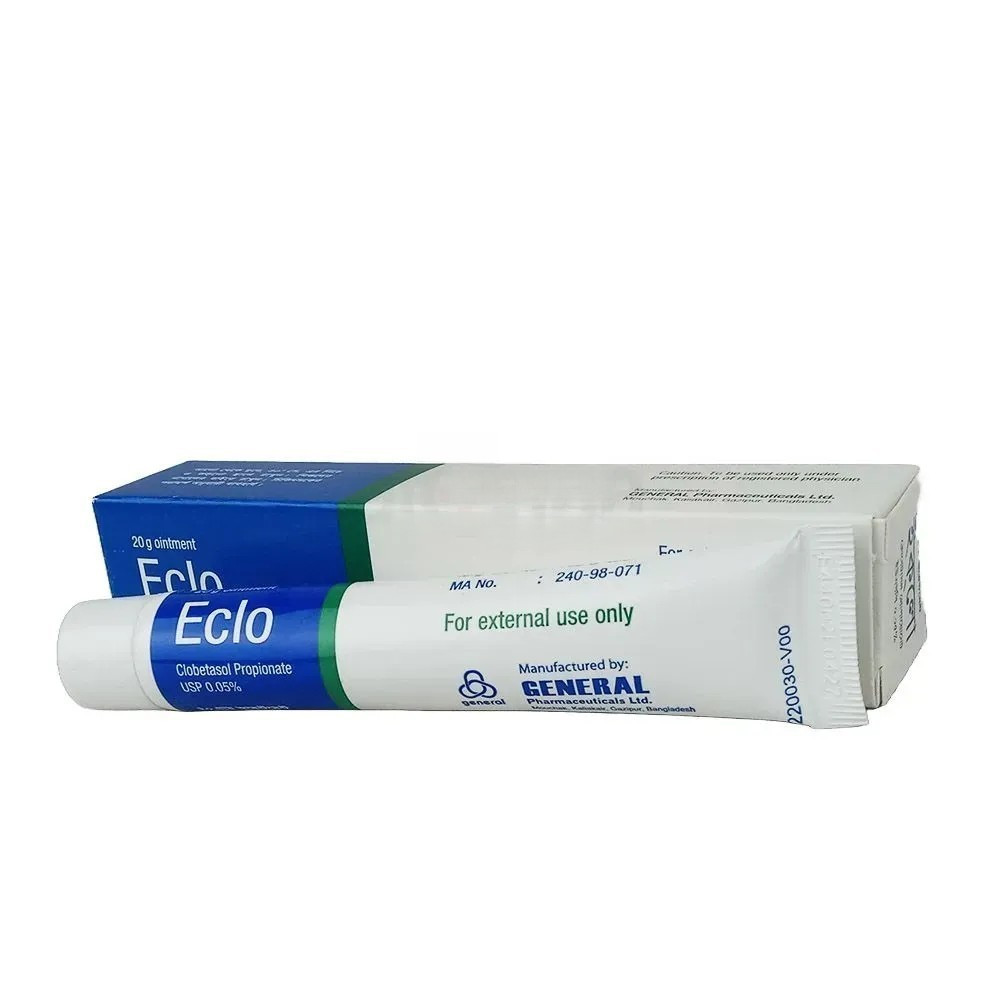

Eclo Ointment 20 gm tube, Clobetasol Propionate 0.05%
Inhouse product
-
৳11.40
৳12.00 -
৳42.75
৳45.00 -
৳16.63
৳17.50 -
৳2.14
৳2.25
Reviews & Ratings
Indications
Eclo is indicated for
adults, elderly and children over 1 year in following dermatoses.
- Psoriasis (excluding widespread
plaque psoriasis)
- Recalcitrant dermatoses
- Lichen planus
- Discoid lupus erythematosus
- Other skin conditions which do
not respond satisfactorily to less potent steroids.
* রেজিস্টার্ড চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন'
Composition
Clobetasol Propionate
Cream: Each gram cream
contains Clobetasol Propionate BP 0.5 mg (0.05% w/w).
Clobetasol Propionate
Ointment: Each gram ointment
contains Clobetasol Propionate BP 0.5 mg (0.05% w/w).
Clobetasol Propionate
Scalp Application: Each gram lotion
contains Clobetasol Propionate BP 0.5 mg (0.05% w/w).
Clobetasol Propionate
Shampoo: Each gram shampoo
contains Clobetasol Propionate BP 0.5 mg (0.05% w/w).
Clobetasol Propionate
Lotion: Each gram lotion
contains Clobetasol Propionate BP 0.5 mg (0.05% w/w).
Clobetasol Propionate
Spray: Each gram spray
contains Clobetasol Propionate BP 0.5 mg (0.05% w/w).
Pharmacology
Clobetasol Propionate
is a very potent topical corticosteroid. It has anti-inflammatory, antipruritic
and vasoconstrictive properties. It shows anti-inflammatory activity via
multiple mechanisms to inhibit late phase allergic reactions. It decreases the
density of mast cells, chemotaxis and activation of eosinophils. It also
reduces cytokine production and inhibits the metabolism of arachidonic acid.
Dosage
Cream, Ointment: Adults, elderly and children over 1 year:
Apply a thin layer of Clobetasol Propionate Cream or Ointment to the affected
skin areas twice daily and rub in gently and completely. Repeated short courses
of Clobetasol Propionate may be used to control exacerbations. In more
resistant lesions, especially where there is hyperkeratosis, the effect of
Clobetasol can be enhanced, if necessary, by occluding the treatment area with
polythene film. Overnight occlusion only is usually adequate to bring about a
satisfactory response.
Clobetasol Propionate is super-high potency topical corticosteroids; therefore,
treatment should be limited to 2 consecutive weeks. The maximum weekly dose
should not be exceeded 50 gm/week. In case of children, courses should be
limited if possible to five days and reviewed weekly.
Spray: Apply required quantity of spray of once or
twice daily to the affected areas of the scalp and gently rub in. The total
dose applied should not exceed 50 ml weekly. If necessary, Clobetasol
Propionate scalp solution may be massaged into the scalp using the tips of the
fingers. Therapy should be discontinued if no response is noted after one week
or as soon as the lesion heals. It is advisable to use Clobetasol Propionate
scalp solution for brief periods only.
Shampoo: It should be applied to the dry (not wet)
scalp once a day to the affected areas only. It should be massaged gently into
the lesions and left in place for 15 minutes before lathering and rinsing.
Treatment should be limited to 4 consecutive weeks. Total dosage of shampoo
should not exceed 50 g per week. Under 18 years this preparation is not
recommended.
Scalp Solution: Apply required quantity of spray of
Clobetasol Scalp Solution once or twice daily to the affected areas of the
scalp and gently rub in. The total dose applied should not exceed 50 ml weekly.
If necessary, Clobetasol Scalp Solution may be massaged into the scalp using
the tips of the fingers. Therapy should be discontinued if no response is noted
after one week or as soon as the lesion heals. It is advisable to use
Clobetasol Scalp Solution for brief periods only.
* রেজিস্টার্ড চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন'
Administration
Route of
administration: Cutaneous. Creams are especially appropriate for moist or
weeping surfaces. Ointments are especially appropriate for dry, lichenified or
scaly lesions.
* রেজিস্টার্ড চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন'
Interaction
Co-administered drugs
that can inhibit CYP3A4 (eg ritonavir, itraconazole) have been shown to inhibit
the metabolism of corticosteroids leading to increased systemic exposure.
Contraindications
- Rosacea, acne vulgaris and
perioral dermatitis. Primary cutaneous viral infections (e.g. herpes
simplex, chickenpox).
- Hypersensitivity to the
preparation.
- The use of Clobetasol
Propionate skin preparations is not indicated in the treatment of
primarily infected skin lesions caused by infection with fungi (e.g.
candidiasis, tinea), or bacteria (e.g.impetigo): perianal and genital
pruritus.
- Dermatoses in children under
one year of age, including dermatitis and napkin eruptions.
Side Effects
- As with other topical
corticosteroids prolonged use of large amounts, or treatment of extensive
areas can result in sufficient systemic absorption to produce the features
of hypercorticism.
- Prolonged and intensive
treatment with a highly active corticosteroid preparation may cause local
atrophic changes in the skin such as thinning, striae and dilatation of
the superficial blood vessels, particularly when occlusive dressings are
used or when skin folds are involved.
- In rare instances, treatment of
psoriasis with corticosteroids (or its withdrawal) is thought to have
provoked the pustular form of the disease.
- There are reports of
pigmentation changes and hypertrichosis with topical steroids. Eclo is
usually well tolerated, but if signs of hypersensitivity appear,
application should be stopped immediately. Exacerbation of symptoms may
occur.
Pregnancy &
Lactation
There are limited data
from the use of Clobetasol Propionate cream in pregnant women. Topical
administration of corticosteroids to pregnant animals can cause abnormalities
of foetal development. The relevance of this finding to humans has not been
established. However, the administration of Clobetasol Propionate Cream during
pregnancy and lactation should only be considered if the expected benefit to the
mother outweighs the possible risks of treatment.
It is unknown whether this drug is excreted in human milk. Because many drugs
are excreted in human milk, caution should be exercised when Clobetasol
Propionate Cream is administered to a nursing woman.
Precautions &
Warnings
Long-term continuous
topical therapy should be avoided where possible, particularly in infants and
children, as adrenal suppression can occur readily even without occlusion. If
used in childhood or on the face, courses should be limited if possible to five
days and occlusion should not be used.
The face, more than other areas of the body, may exhibit atrophic changes after
prolonged treatment with potent topical corticosteroids. This must be borne in
mind when treating such conditions as psoriasis, discoid lupus erythematosus
and severe eczema.
If applied to the eye lids, care is needed to ensure that the preparation does
not enter the eye, as glaucoma or cataract might result.
Topical corticosteroids may be hazardous in psoriasis for a number of reasons
including rebound relapses, development of tolerance, risk of generalised
pustular psoriasis and development of local or systemic toxicity due to
impaired barrier function of the skin. If used in psoriasis careful patient
supervision is important.
Appropriate anti-microbial therapy should be used whenever treating
inflammatory lesions which have become infected. Any spread of infection
requires withdrawal of topical corticosteroid therapy and systemic
administration of anti-microbial agents. Bacterial infection is encouraged by
the warm, moist conditions induced by occlusive dressings, and so the skin
should be cleansed before a fresh dressing is applied.
Use in Special
Populations
In infants and
children under 12 years of age, long-term continuous topical corticosteroid
therapy should be avoided where possible, as adrenal suppression can occur.
Children are more susceptible to the use of topical corticosteroids which
develops atrophic changes.
Overdose Effects
Acute overdosage is
very unlikely to occur, however, in the case of chronic over-dosage or misuse
the features of hypercortisolism may occur and in this situation topical
steroid should be discontinued.
Therapeutic Class
Other Topical
corticosteroids
Storage Conditions
Keep below 30°C
temperature, protected from light and moisture. Do not freeze. Keep out of the
reach of children.
Frequently Bought Products
Lipitin Tablet, Atorvastatin Calcium 40 mg
Famodin Tablet (15 tablets Strip), Famotidine 20 mg
Rutix Tablet, Ofloxacin 400 mg
Olmecar Tablet, Olmesartan Medoxomil 20 mg
Dirozyl Tablet (10 Tablets Strip), Metronidazole 400 mg
Product Queries (0)
Login Or Registerto submit your questions to seller
Other Questions
No none asked to seller yet
-
৳11.40
৳12.00 -
৳42.75
৳45.00 -
৳16.63
৳17.50 -
৳2.14
৳2.25










![Acme's ORS Oral Powder, Oral rehydration salt [glucose based] 10.25 gm](https://www.skpharma.com.bd/public/uploads/all/BfReBR7TDfIW2VeGN8AIe4bNFSgGlLGUAHrixSRd.webp)



