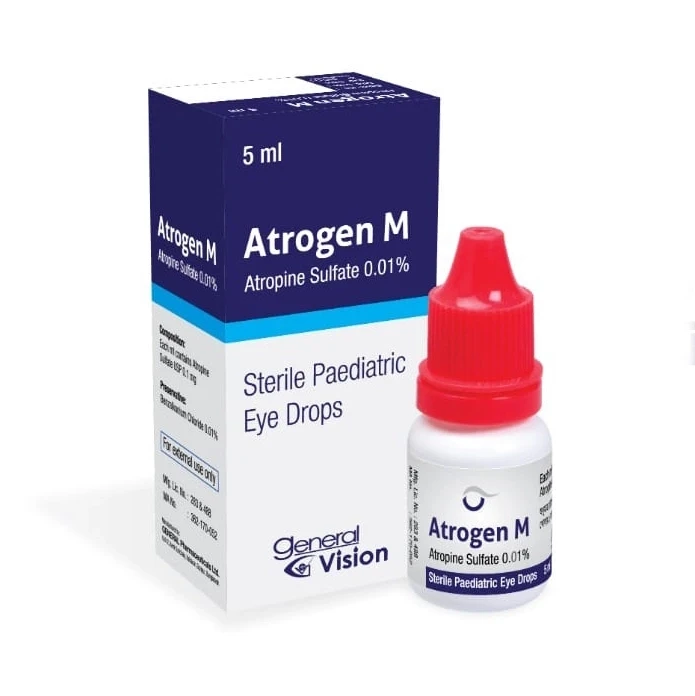

Atrogen M Ophthalmic Solution 5 ml drop, Atropine Sulfate 0.01%
Inhouse product
-
৳11.40
৳12.00 -
৳42.75
৳45.00 -
৳16.63
৳17.50 -
৳2.14
৳2.25
Reviews & Ratings
Indications
1% ophthalmic
preparation: As a cycloplegic or
mydriatic for refraction or for desired dilatation of the iris or uveal tract
during acute infammatory conditions.
0.01% ophthalmic
preparation: This medication is
indicated as a treatment to slow the progression of myopia in children aged
from 4 to 14 years. Atrogen M treatment may be initiated in children when
myopia progresses.
* রেজিস্টার্ড চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন'
Composition
1% ophthalmic
preparation: Each ml sterile eye
drops contains Atropine Sulphate BP 10 mg. Preservative: Benzalkonium Chloride
0.01%
0.01% ophthalmic
preparation: Each ml sterile eye
drops contains Atropine Sulphate USP 0.1 mg. Preservative: Sodium Perborate
0.01%
Pharmacology
The exact mode of
action of Atropine Sulfate is attributed to the suppression of the progression
of myopia. Muscarinic receptors are widely distributed in ocular tissues, with
roles in ocular growth and development, as well as accommodation, recognized.
Preclinical studies suggest that Atropine Sulfate acts via binding to the
muscarinic receptors located on scleral fibroblasts and possibly in the retina,
primarily the M1, M3 and M4 subtypes. This results in changes in the activity
of cell signaling proteins and enzymes such as MEK-ERK-MAPK and
transglutaminases, and possibly dopamine release, causing scleral remodeling or
strengthening, leading to a reduction in axial length and vitreous chamber
depth and consequently a suppression of myopia progression.
Dosage &
Administration
1%
ophthalmic preparation-
- For uveitis: One drop three
times daily
- For refraction: One drop in the
eyes twice daily for one or two days before examination, and one hour
before examination. Pressure should be maintained over lacrimal sac,
occluding lacrimal duct for one minute after instillation.
0.01% ophthalmic
preparation- This paediatric eye
drops should be administered as one drop to each eye at night. The maximum
benefit of treatment may not be achieved with less than a 2-year continued
administration period. To minimize the risk of systemic absorption, gentle
pressure should be applied to the tear duct for one minute after application.
* রেজিস্টার্ড চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন'
Interaction
Atrogen M should not
be used with the following medications, because serious interactions may occur
with Anticholinergics, Antiglaucoma agents, Potassium Citrate, Potassium
Supplements, Carbachol, Physostigmine or Pilocarpine & CNS medication.
Contraindications
This eye drops is
contraindicated in the presence of angle closure glaucoma or where angle
closure glaucoma is suspected. If used in glaucoma susceptible patients, an
estimation of the depth of the angle of the anterior chamber should be
performed prior to the initiation of therapy.
Side Effects
Blurred vision, local
irritation, follicular conjunctivitis, vascular congestion, oedema, exudate,
contact dermatitis, eczematous dermatitis, photophobia etc.
Pregnancy &
Lactation
Atropine Sulphate may
be systemically absorbed after ocular administration; however, signifcant
efects on the fetus have not been reported. Systemically absorbed Atropine
Sulphate is distributed into breast milk in very small amounts. It may cause
adverse efects, such as rapid pulse, fever, or dry skin, in nursing infant of
mothers using ophthalmic Atropine.
Precautions &
Warnings
Atrogen M should not
be used in children who have previously had severe systemic reaction to Atrogen
M. An increased susceptibility to Atrogen M has been reported in children with
Down’s syndrome, spastic paralysis, or brain damage; therefore Atrogen M should
be used with great caution in these patients. It is recommended that regular
eye health clinical reviews are conducted if long term treatment is planned,
including the monitoring of anterior segment development, IOP, retinal health
and myopia progression. This eye drops should not be used in children less than
4 years of age.
Overdose Effects
Flush the eye with
water and seek emergency medical attention. Symptoms of a atropine ophthalmic
overdose include headache, fast heartbeat, dry mouth and skin, unusual
drowsiness, and fushing.
Therapeutic Class
Mydriatic and
Cycloplegic agents
Storage Conditions
Store below 30°C and
dry place, away from light. Keep out of the reach of children, do not touch the
tip of dropper bottle since this may contaminate the product. Do not use the
eye drops after 1 month of first opening of the dropper.
Frequently Bought Products
Vonity Tablet, Vonoprazan 10 mg
Venlax Tablet, Venlafaxine Hydrochloride 37.5 mg
Deflacort Oral Suspension, Deflazacort 6 mg/5 ml, 60ml
Tamoxen Tablet, Tamoxifen Citrate 20 mg
Product Queries (0)
Login Or Registerto submit your questions to seller
Other Questions
No none asked to seller yet
-
৳11.40
৳12.00 -
৳42.75
৳45.00 -
৳16.63
৳17.50 -
৳2.14
৳2.25